Can infections lead to cancer?
- Coach Chuck
- Jun 21, 2021
- 6 min read
Updated: Jun 21, 2022

Scientific recognition of the connection between infections and cancer has risen remarkably since the beginning of the 21st century.(1) Although previous investigations first linked H. pylori infection to gastric cancer over four decades ago, the association between these phenomena appeared limited and unclear.(2) The latent period between carcinogenic infections and cancer can span decades, which substantially complicates researchers’ efforts to evaluate causality and the efficacy of control strategies.(1) That said, epidemiological survey data that has accumulated over time now decisively validates the causative role of chronic infections in cancer. According to one assessment, 2.2 million new cancer cases traced their origin to infections in 2018, which equals 13% of the world’s cancer burden. The distribution of carcinogenic infections varies vastly by geographic locale, with such disease being highest among populations of developing countries in East Asia and Sub-Saharan Africa.(3) These disparities in prevalence arise from a combination of factors, most notably deficits in medical infrastructure, public sanitation, and vaccine availability.(3,4)
Of the 11 biological agents identified as group 1 carcinogens by the International Agency for Research on Cancer (IARC), 4 account for upwards of 90% of infection-mediated cancers: H. pylori, high-risk human papillomavirus (HPV), hepatitis B virus (HBV), and hepatitis C virus (HCV).(4) Both men and women equally share the burden of infection-mediated cancers, but causative pathogens vary markedly by sex:
Table 1: Estimated numbers of infection-attributable cancer cases in 2018, by infectious pathogen, cancer subsite, and biological gender. Adapted from de Martel, et al. (2018):

The pathogenesis of infection-mediated cancer differs depending upon the infectious agent. In patients with hepatitis B, hepatic inflammation and injury seen in the disease arises from the host’s own immune response. Over time, this chronic, underlying inflammation generates a mitogenic, mutagenic cellular environment that can promote hepatocarcinogenesis.(5) Researchers hypothesize that a minimum of two mechanisms drive HBV-mediated hepatocarcinoma. The first is integration of the viral genome into host cellular DNA, thereby disrupting proper expression of cellular genes. The second is expression of HBV proteins, which can alter signaling pathways that mediate inflammatory and oncogenic pathways.(6) Other viruses, like Kaposi sarcoma herpes virus, modulate autophagy as well as oncogene-induced senescence.(7) While work continues to clarify the oncogenic mechanisms in HCV, researchers have uncovered the oncoproteins (E5, E6, E7) responsible for cancer development in HPV.(8) Taken together, these discoveries may facilitate the development of treatments that can specifically target these carcinogenic bioagents.
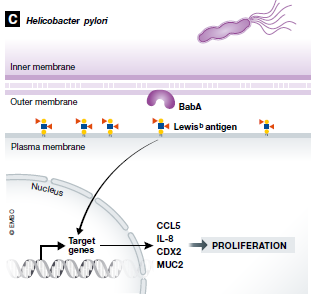
Unlike viruses, which propagate inside hijacked host cells, bacteria like Heliobacter pylori promote cancer development through extracellular means. H. pylori bacteria express CagL – an adhesin – that binds β5-integrin present on the exterior surface of gastric epithelial cells. This union results in downstream activation of kinase complexes that induce gastrin secretion and development of hypergastrinemia.(9) In addition to CagL, H. pylori also bind epithelial EGFR via OipA, as well as Lewis (b) surface epitopes through BabA, another adhesin protein. Activation of EGFR leads to cancerous Akt and β-catenin signaling, whereas binding of the Lewis (b) surface epitopes results in increased mRNA levels of proinflammatory cytokines and precancer-related factors.(10,11) As a Gram-negative bacterium, H. pylori also cover their exterior surface macromolecules with polysaccharide-rich capsules, which shield structures that can otherwise trigger the complement cascade.(9) To further evade detection by either arm of the immune system, H. pylori display modified lipopolysaccharides, flagella, and peptidoglycans that are poor substrates for immune receptors (e.g., TLR4).(12) Continued colonization of the gut by H. pylori can raise the risk of peptic ulcer disease, gastric adenocarcinoma, and mucosa-associated lymphoid tissue lymphoma. Rather alarmingly, half of the world’s population is estimated to have an H. pylori infection, which makes them candidates for chronic inflammation.(2)
Figure 1: Heliobacter pylori (H. pylori) expresses several extracellular proteins that interact with gastric cell surface components. These interactions trigger signaling cascades that result in cellular malignancy. Adapted from van Elsland et al. (2018):
Parasitic infestations and fungal infections can also greatly elevate the risk of developing certain cancers. According to data from WHO, approximately 1.5 billion people in the world have roundworms, making it the most common infection worldwide.(13) Around 1.3 billion have hookworm and 265 million are believed to have schistosome parasitic infections.(14) Since the mid-20th century, medical professionals have observed a robust positive correlation between the prevalence of bladder squamous cell carcinoma and urinary schistosomiasis.(15) While scientific understanding of the causative carcinogenic mechanism of action remains incomplete, researchers hypothesize that parasitic-derived catechol estrogens may be to blame. When metabolized, these estrogens form reactive oxygen species that can damage DNA.(16) Exogenous, parasitic eggs may also trigger the formulation of granulomas and sustain chronic inflammation.(13) In a similar manner, fungal strains like Malassezia appear to drive the formation of pancreatic ductal adenocarcinoma by activating mannose-binding lectin, which in turn drives the complement cascade. Investigators studying the phenomenon noticed that ablation of the fungal mycobiome confers protection against tumor growth, as well as extensive colocalization and infiltration of Malassezia spp. in PDA tumors.(17) Continued study of the mycobiome, parasites, and infectious agents has progressively unveiled a panoply of striking connections to cancer, many of which remain understudied.(18)
Although the link between infection and cancer appears like an unwelcome development in oncology, this discovery may help improve patient outcomes going forward. As modifiable risk factors, infections can be prevented if healthcare systems implement policies that limit transmission of communicable, disease-causing pathogens.(19) In particular, global scaling of vaccination efforts against viruses like HPV can close the current prevalence gap seen between the developed and developing world.(20) To combat entities for which no vaccine exists, like H. pylori, clinicians may want to explore the use of antibiotics to control bacterial growth in the gastrointestinal tract. While researchers continue to debate the merits and consequences of antibiotic use on the microbiome, this overlooked strategy could assist ongoing efforts to dampen the incidence of gastric cancer.(3) If prophylactic measures were completely successful in eradicating infections, cancer cases in the developing world would plummet by 1,000,000 per year and by 375,000 in the developed world.(5)
Stay strong, keep smiling and be your own best doctor,
- Chuck
Charles J. Meakin MD, MHA, MS
Disclaimer: This information is not meant as direct medical advice. Readers should always review options with their local medical team. This is the sole opinion of Dr. Meakin based on a literature review at the time of the blog and may change as new evidence evolves.
1 Baussano I, Franceschi S, Plummer M. Infection transmission and chronic disease models in the study of infection-associated cancers. Br J Cancer. 2014;110(1):7-11.
2 Zhu C, Wang Y, Cai C, et al. Bacterial infection and associated cancers. Adv Exp Med Biol. 2017;1018:181-191.
3 de Martel C, Georges D, Bray F, et al. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;8:e180-e190.
4 Plummer M, de Martel C, Vignat J, et al. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4:e609-e616.
5 Oh JK, Weiderpass E. Infection and cancer: global distribution and burden of diseases. Ann Glob Health. 2014;80(5):384-392.
6 Brechot C, Kremsdorf D, Soussan P, et al. Hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC): molecular mechanisms and novel paradigms. Pathologie-biologie. 2010;58(4):278-287.
7 Leidal AM, Cyr DP, Hill RJ, et al. Subversion of autophagy by Kaposi’s sarcoma-associated herpesvirus impairs oncogene-induced senescence. Cell Host Microbe. 2012;11:167-180.
8 Ghittoni R, Accardi R, Hasan U, et al. The biological properties of E6 and E7 oncoproteins from human papillomaviruses. Virus Genes. 2010;40:1-13.
9 Van Elsland D, Neefjes J. Bacterial infections and cancer. EMBO Rep. 2018;19(11):e46632.
10 Polk DB, Peek RM Jr. Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10:403-414.
11 Ishijima N, Suzuki M, Ashida H, et al. BabA-mediated adherence is a potentiator of the Helicobacter pylori type IV secretion system activity. J Biol Chem. 2011;286:25256-25264.
12 Tran AX, Stead CM, Trent MS. Remodeling of Helicobacter pylori lipopolysaccharide. J Endotoxin Res. 2005;11:161-166.
13 World Health Organization. WHO recommends large-scale deworming to improve children’s health and nutrition. https://www.who.int/news/item/29-09-2017-who-recommends-large-scale-deworming-to-improve-children-s-health-and-nutrition. Published September 29, 2017. Accessed June 11, 2021.
14 Jockers D. Parasite infections: functional lab analysis to identify parasites. https://drjockers.com/parasite-infections/. Published October 2020. Accessed June 11, 2021.
15 Berry A, Iriart X, Fillaux J, et al. Urinary schistosomiasis and cancer. Bull Soc Pathol Exot. 2017;110(1):68-75.
16 Botelho MC, Alves H, Richter J. Halting Schistosoma haematobium – associated bladder cancer. Int J Cancer Manag. 2017;10(9):e9430.
17 Aykut B, Pushalkar S, Chen R, et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature. 2019;574:264-267.
18 Knoll LJ, Hogan DA, Leong JM, et al. Pearls collections: what we can learn about infectious disease and cancer. PLOS Pathog. 2018;14(3):e1006915.
19 Gredner T, Behrens G, Stock C, et al. Cancers due to infection and selected environmental factors. Dtsch Arztebl Int. 2018;115(35-36):586-593.
20 Hanson CM, Eckert I, Bloem P, et al. HPV programs: application to implementation. Vaccines (Basel). 2015;3:408-419.







Comments